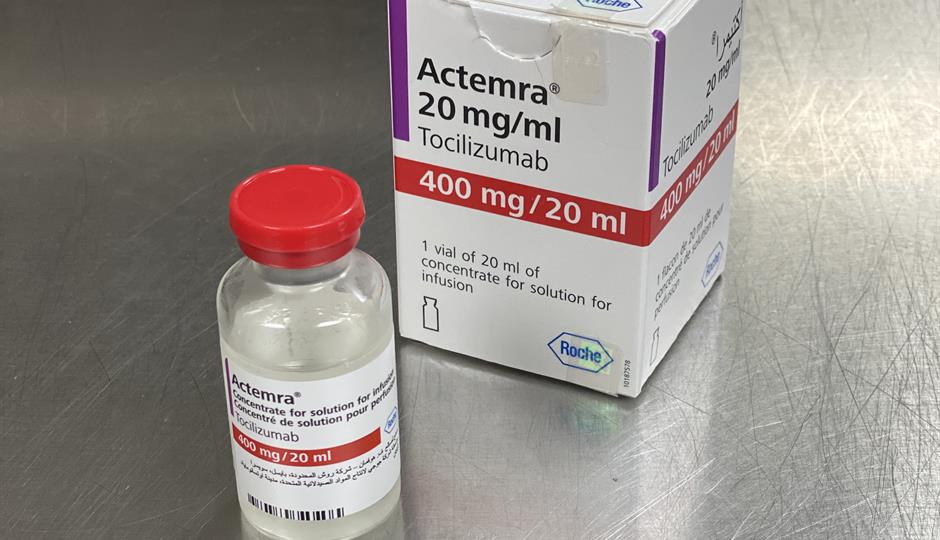
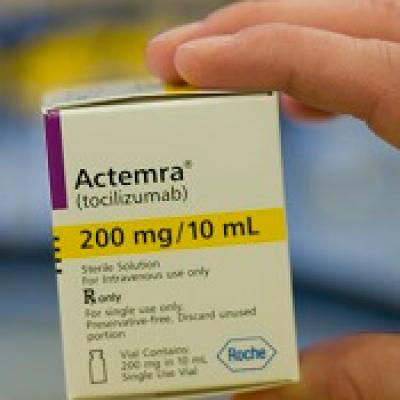
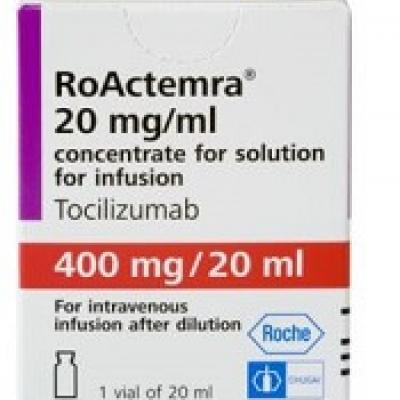
Tocilizumab Injection Supply: Delhi HC asks Centre to hold meeting with Roche to ensure immediate supply of Tocilizumab - The Economic Times
Approval lapsed) RoActemra tocilizumab 80mg/4mL concentrate for solution for infusion (Roche Germany) | Therapeutic Goods Administration (TGA)
Approval lapsed) RoActemra tocilizumab 200 mg/ 10mL concentrate for solution for infusion (Roche Germany) | Therapeutic Goods Administration (TGA)
Approval lapsed) RoActemra tocilizumab 400 mg/ 20mL concentrate for solution for infusion (Roche Germany) | Therapeutic Goods Administration (TGA)
/cloudfront-us-east-2.images.arcpublishing.com/reuters/QFWUTH5BLZN5LGWVT27LGWPZBE.jpg)