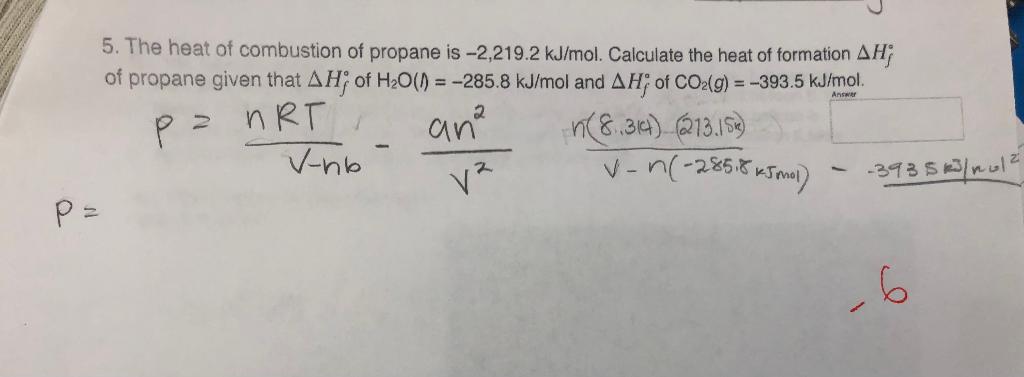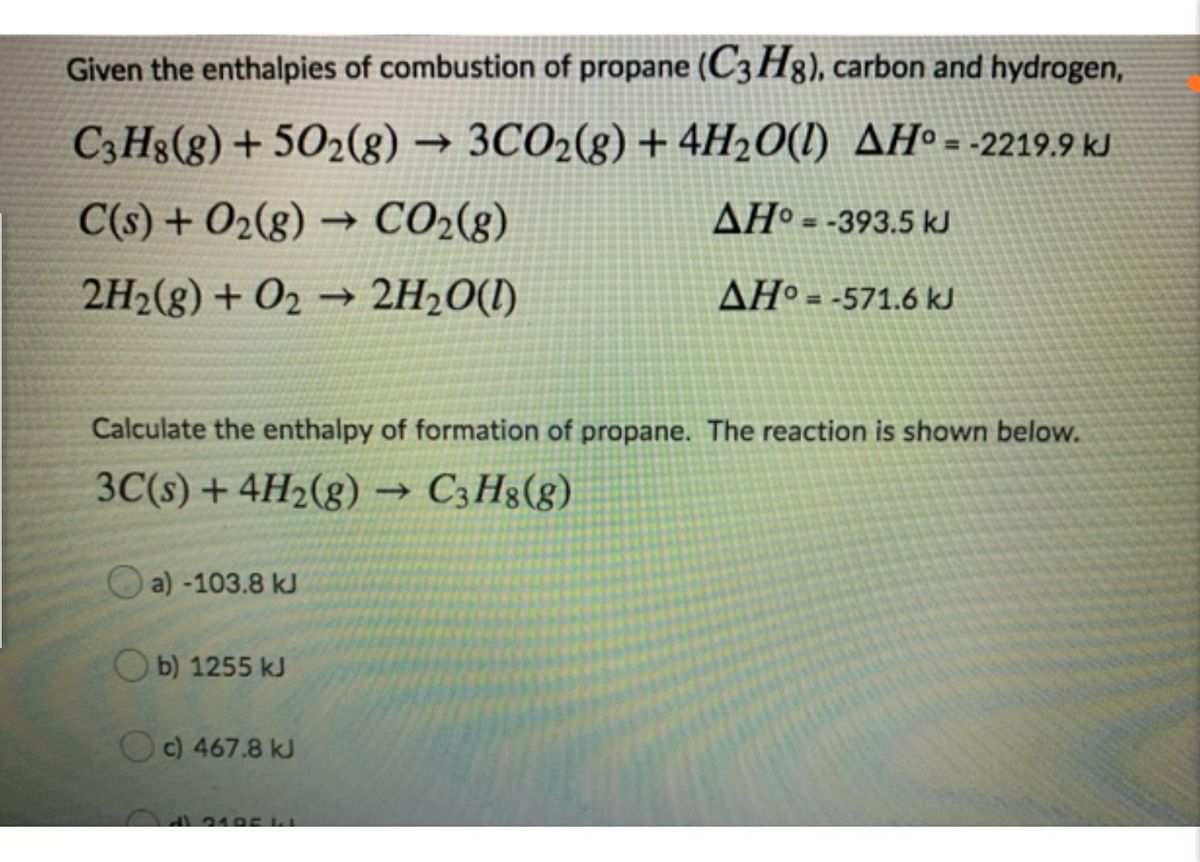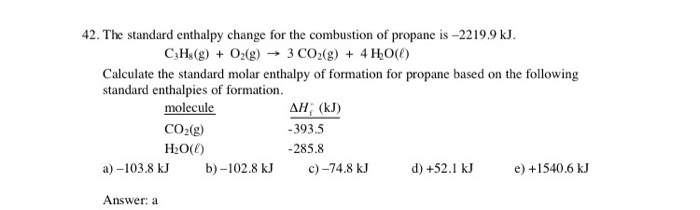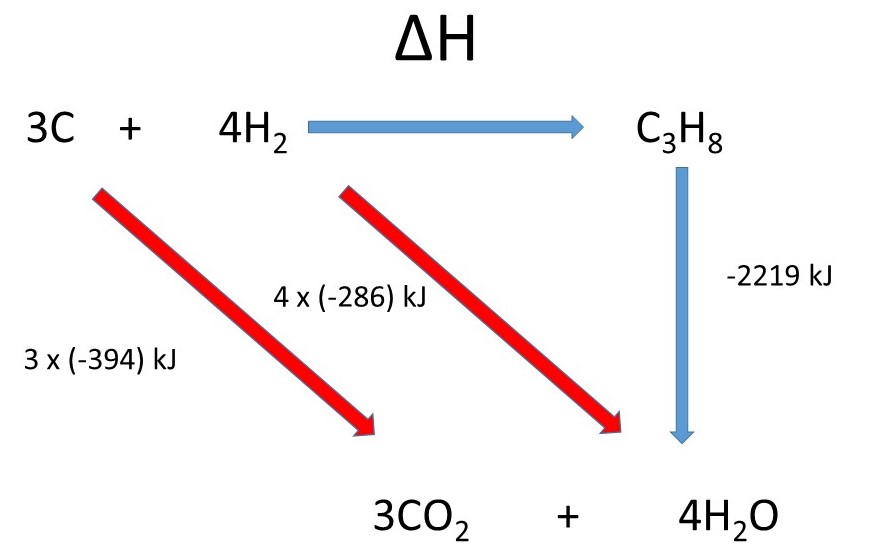
How do you determine the enthalpy change for the reaction below using the enthalpy of combustion data in the table: 3C_((s)) +4H_(2(s)) -> C_3H_(8(s))? | Socratic
![SOLVED: The combustion of propane, C3] Hs , occurs via the reaction C3Hs(g) + 502(9)-3C02(g) + 4Hz O(g) with heat of formation values given by the following table: Substance H? (kJ mol-1 SOLVED: The combustion of propane, C3] Hs , occurs via the reaction C3Hs(g) + 502(9)-3C02(g) + 4Hz O(g) with heat of formation values given by the following table: Substance H? (kJ mol-1](https://cdn.numerade.com/ask_images/b65e98983f40421ab8c3e278181d8324.jpg)
SOLVED: The combustion of propane, C3] Hs , occurs via the reaction C3Hs(g) + 502(9)-3C02(g) + 4Hz O(g) with heat of formation values given by the following table: Substance H? (kJ mol-1

The enthalpy of combustion of propane (C3H8) gas in terms of given data is :Bond energy (kJ/mol) ^εC - H ^ε O = O ^εC = O ^εO - H ^εC -

Calculate the standard enthalpy of formation of propane (C(3)H(8)) if its enthalpy of combustion is -2220.2 kJ "mol"^(-1).The enthalpies of formation of CO(2)(g) and H(2)O(l) are -393.5 and -285.8 kJ "mol"^(-1) respectively.

SOLVED: Consider the combustion of propane, CzH:: CyHele) +5 Ozle) 73 COzle) +4 HzOts) LHSn = -2252 kJ All of the heat from the combustion of a sample of propane goes into

The enthalpy change for the reaction C3H8 (g) + H2 (g) ⟶C2H6 (g) + CH4 (g) at 25C is - 55.7 KJ/mol. Calculate the enthalpy of combustion of C2H6 (g) . The

Following are heats of combustion per mole for methane, propane, and 2,2,4-trimethylpentane. Each is a major source of energy. On a gram-for-gram basis, which of these hydrocarbons is the best source of
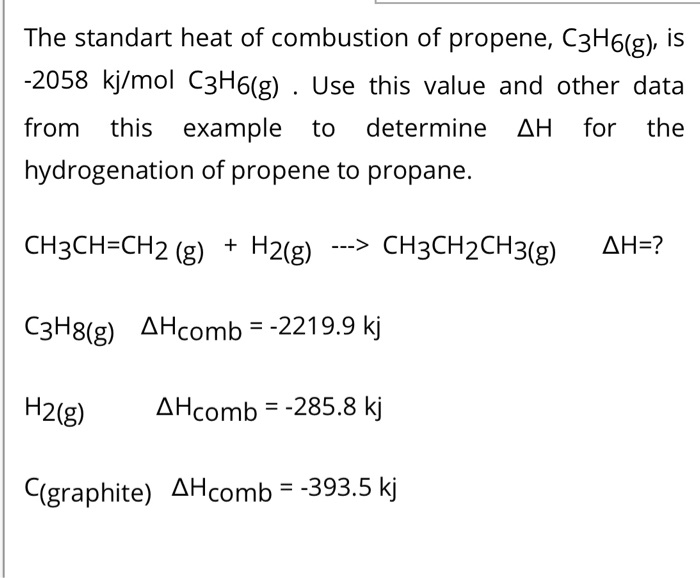
SOLVED: The standart heat of combustion of propene, CzHolg)' is -2058 kjlmol CzHo(g) Use this value and other data from this example to determine AH for the hydrogenation of propene to propane:
Calculate the standard heat of formation of propane, if its heat of combustion is 0-2220.2 KJ mol^-1 the heats of formation - Sarthaks eConnect | Largest Online Education Community

SOLVED: The standard enthalpy of reaction for the combustion of 1 mole of propane is -2666.0 kJ. In this reaction, propane (C3H8(g)) reacts with gaseous O2 to produce CO2(g), H2O(l), and energy

Calculate the heat of combustion (kJ) of propane, C3H8 using the listed standard enthalpy of reaction data: C3H8 (g) + 5O2 (g) ⟶ 3CO2 (g) + 4H2O (g) 3C (s) + 4H2 (