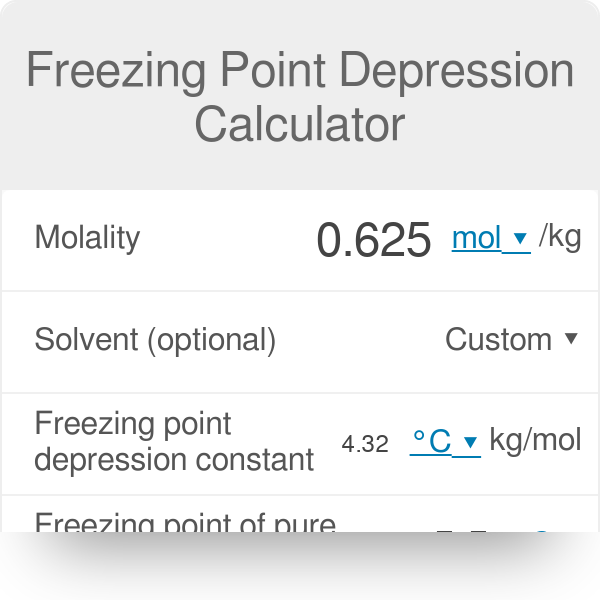
A solution contains 3.22 g of HClO2 in 47.0 g of water. The freezing point of the solution is 271.10 K .Calculate the fraction of HClO2 that undergoes dissociation to H^+ and

Calculate the freezing point depression and boiling point elevation of a solution of `10.0 g` of... - YouTube
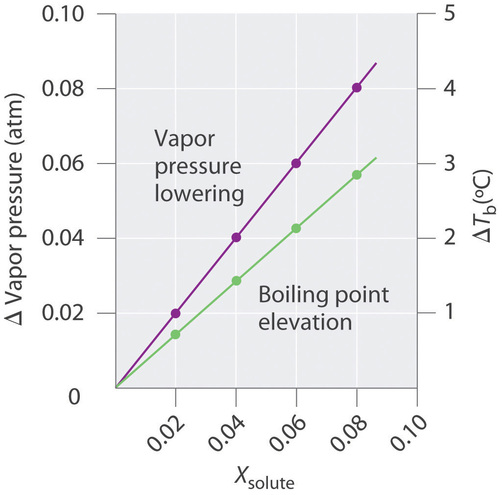
13.8: Freezing-Point Depression and Boiling-Point Elevation of Nonelectrolyte Solutions - Chemistry LibreTexts

Calculate the freezing point and the boiling point at 1 atmosphere of a solution containing 30 g cane sugar (molecular mass 342 ) and 150 g water.Given : Kb = 0.513 and Kf = 1.86
Calculate the freezing point of a solution containing 60 g of glucose (Molar mass = 180 g mol^-1) in 250 g of water. - Sarthaks eConnect | Largest Online Education Community