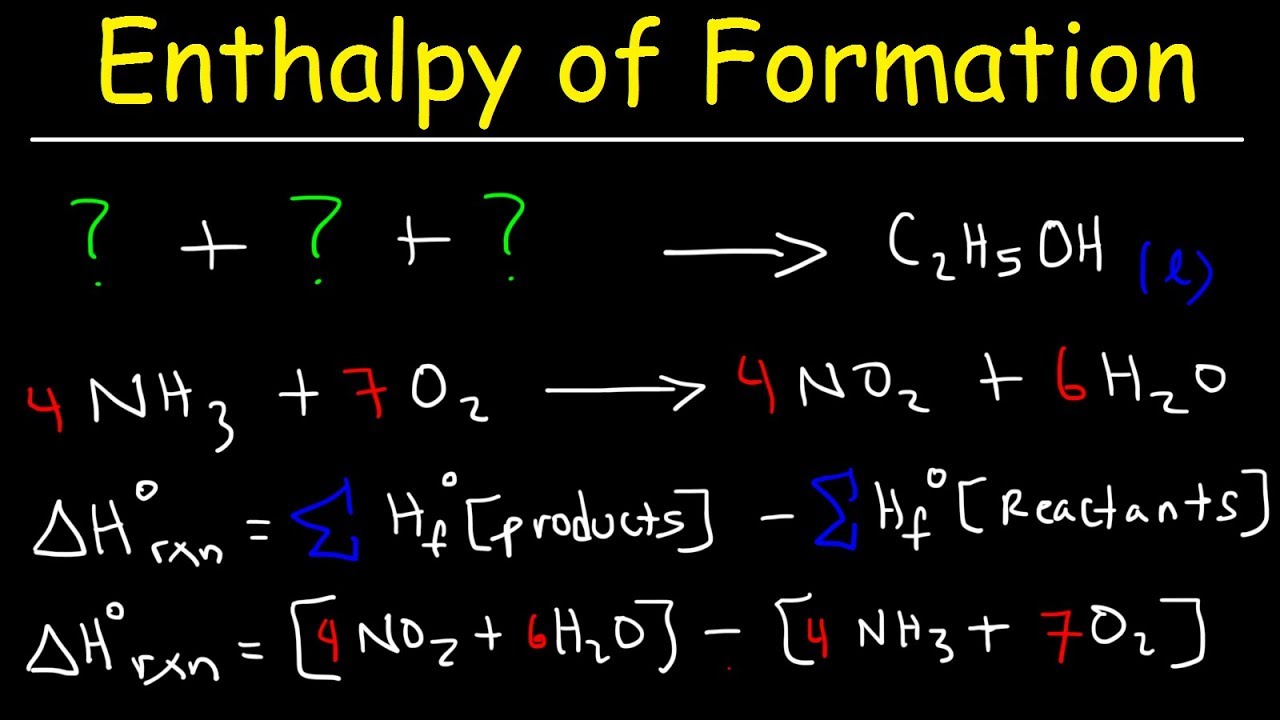
Question Video: Calculating Standard Enthalpy of Combustion of Methane Using Standard Enthalpies of Formation of Methane and Carbon Dioxide | Nagwa

Why does the enthalpy of combustion for alkanes increase, e.g. from methane to propane, etc.? - Quora
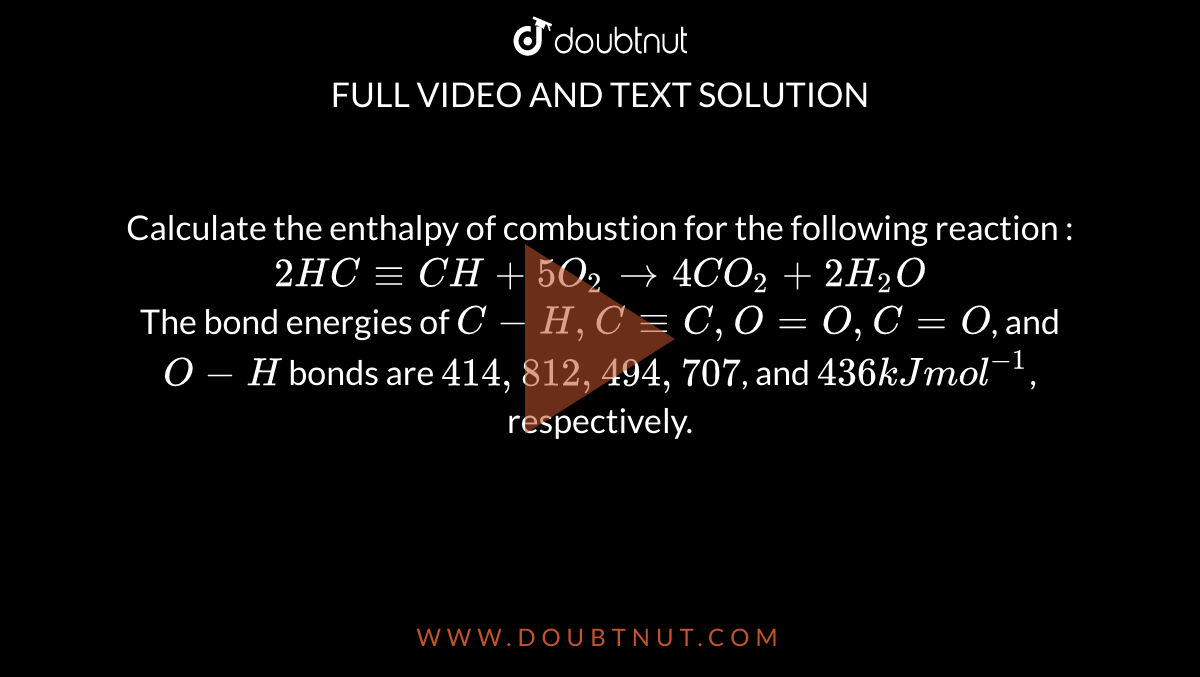
Calculate the enthalpy of combustion for the following reaction : 2HC-= CH +5O(2) rarr 4CO(2) +2H(2)O The bond energies of C-H, C-=C, O=O, C=O, and O-H bonds are 414,812, 494,707, and 436
the enthalpies of combustion of carbon and carbon monooxide are 393.5kJ and 283kJ ,respectively the enthalpy of formation of carbon monoxide 1. 676.5kJ 2. 110.5kJ 3.110.5kJ 4.676.5
combustion of 1 mol of benzene at 298K and 1 atm pressure produces 3267 kJ of heat. Calculate the s†an dard enthalpy of formation of benzene given that the enthalpy of formation

SOLVED: If you know the standard enthalpy of combustion of iron Fe = -416 KJ / mol Calculate the standard enthalpy of combustion of fe203 in the following reaction Afe ( s ) +

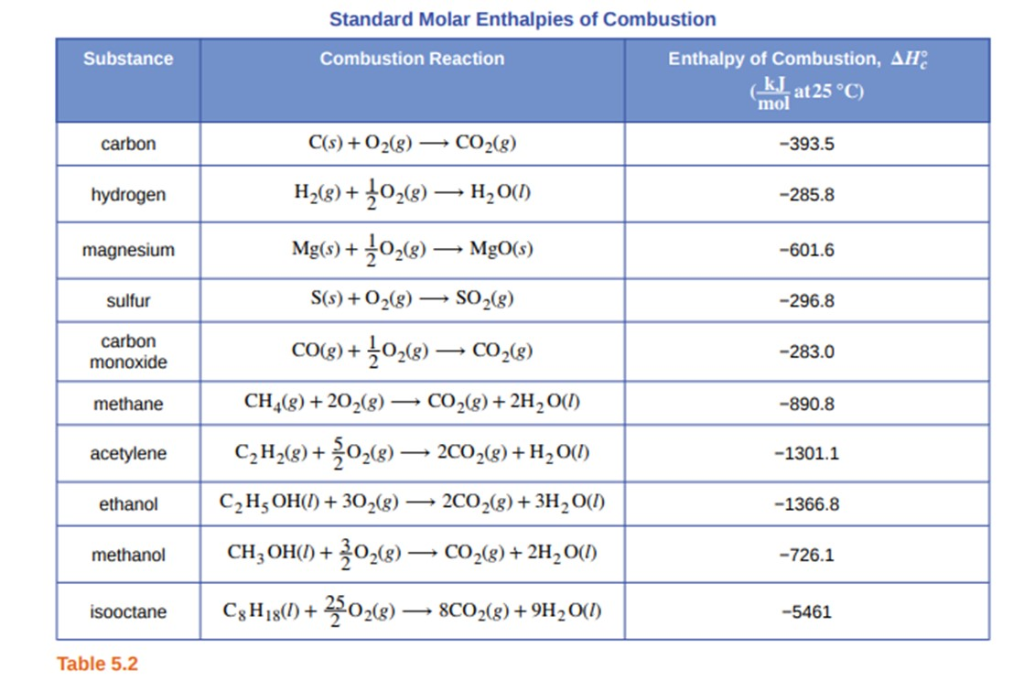
The enthalpy of combustion of methane, graphite, and dihydrogen at 298 K are - 890.3 KJ mol^-1 - 393.5 KJ mol^-1 and - 285.8 KJ mol^-1 respectively. Enthalpy of formation of CH4(g) will be

The enthalpy change for the reaction C(3)H(8)(g)+H(2)(g) rarr C(2)H(6)(g)+CH(4)(g) at 25^(@)C is -55.7 kJ/mol. Calculate the enthalpy of combustion of C(2)H(6)(g). The enthalpy of combustion of H(2), & CH(4) are -285.8 & -

The standard enthalpies of combustion of C6H6 (l) , C (graphite) and H2 (g) are respectively - 3270 kJ mol^-1, - 394 kJ mol^-1 and - 286 kJ mol^-1 . What is