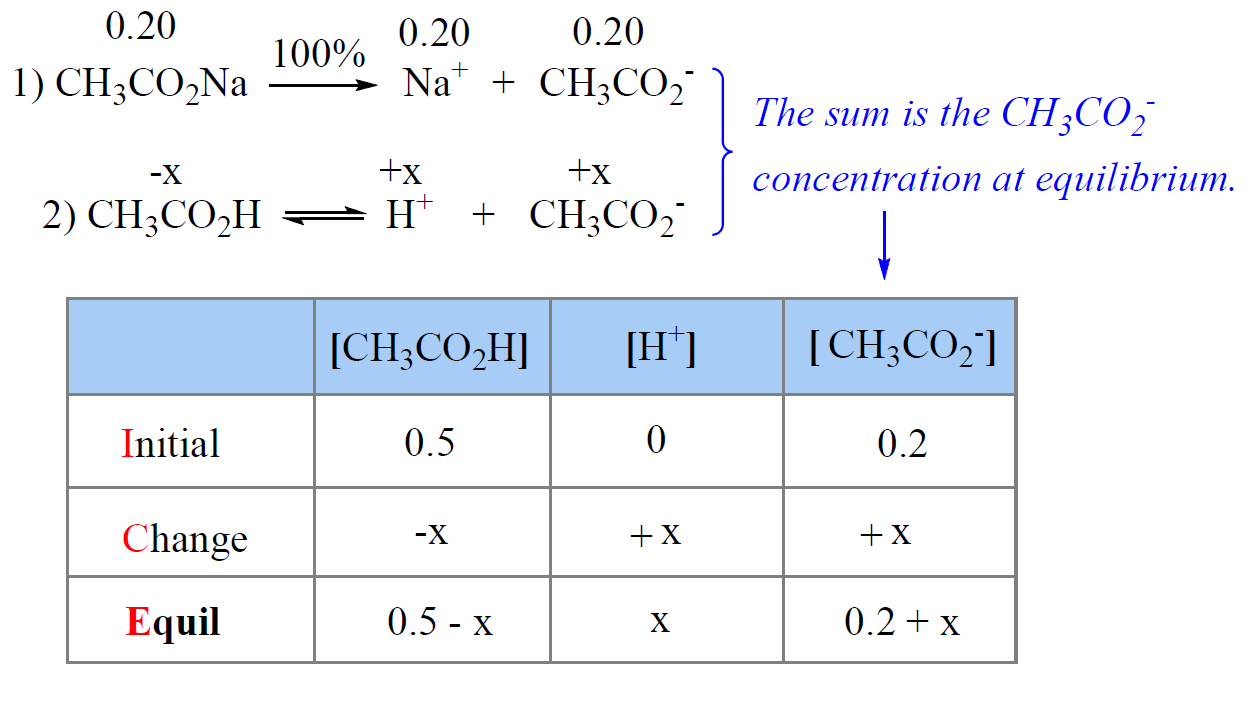
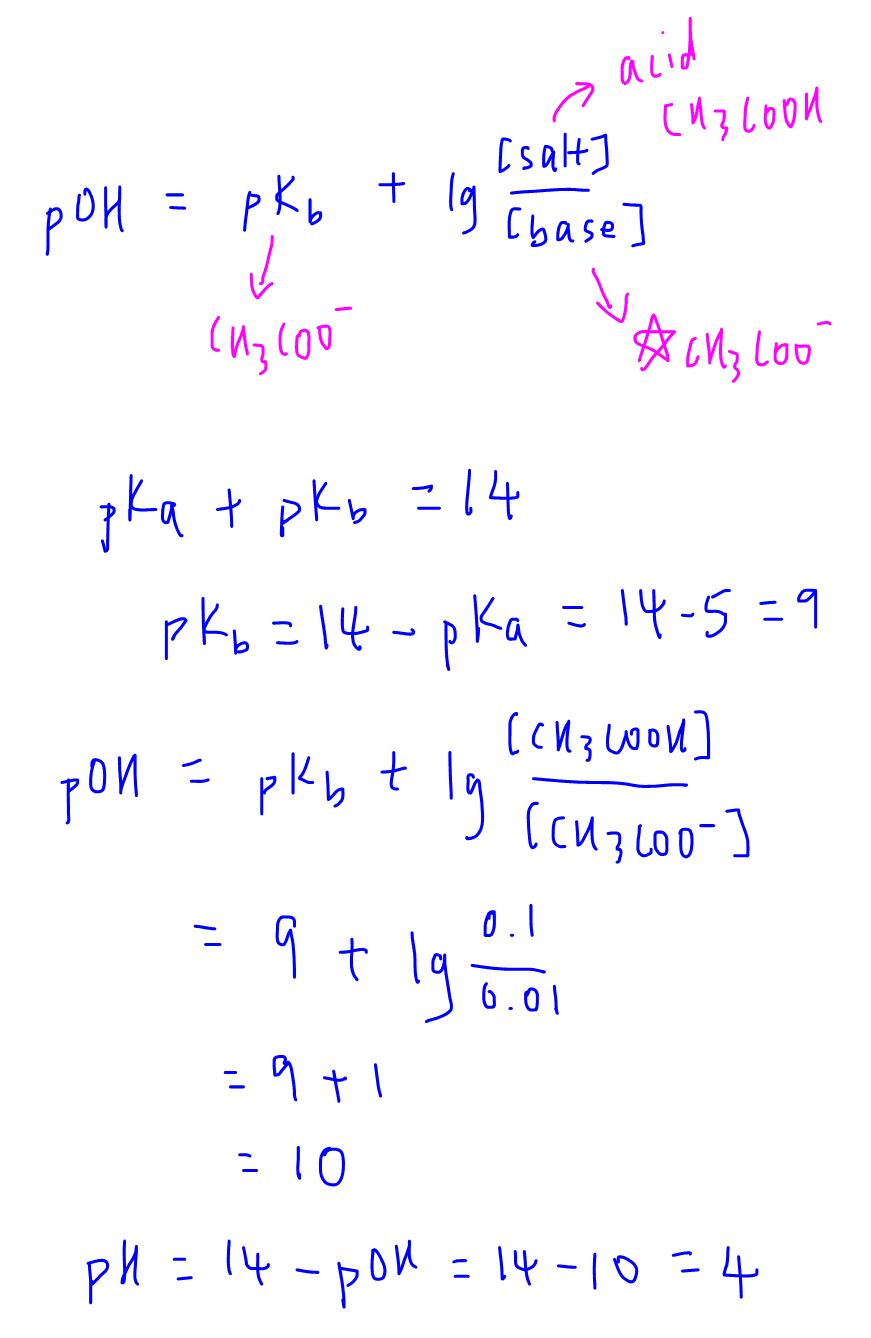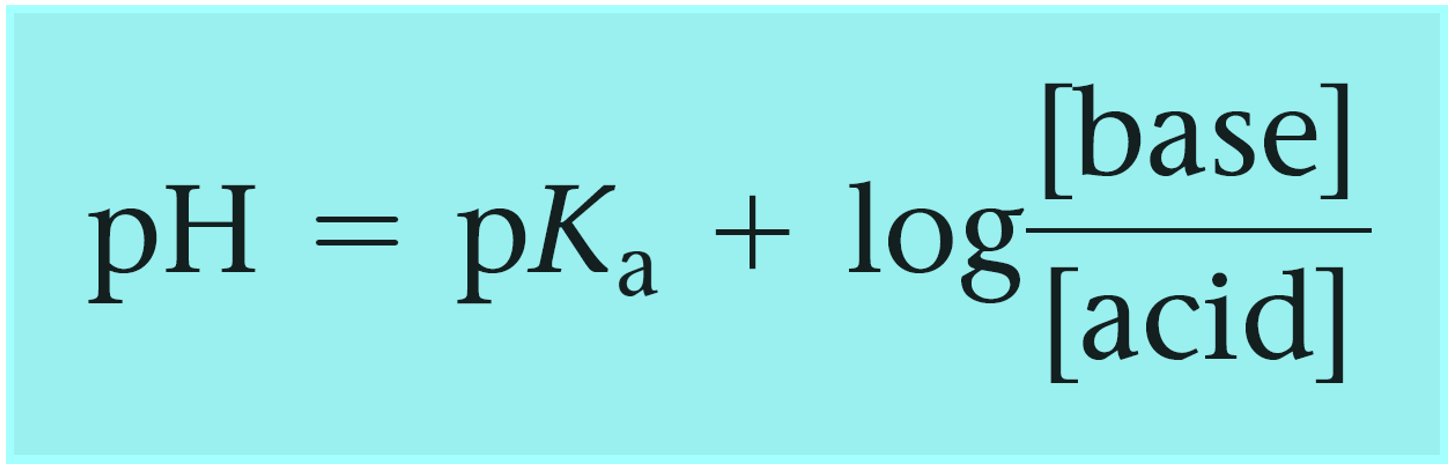SOLVED: B. pH of Buffer Solutions '9. 50 9 Mass of NaCzH;Oz * 3HzO (FW 1365 g/mol) 41 pH of Original buffer solution of Buffer + HCI 4S pH pH Buffer +
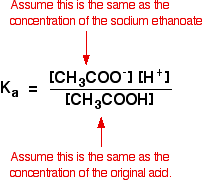
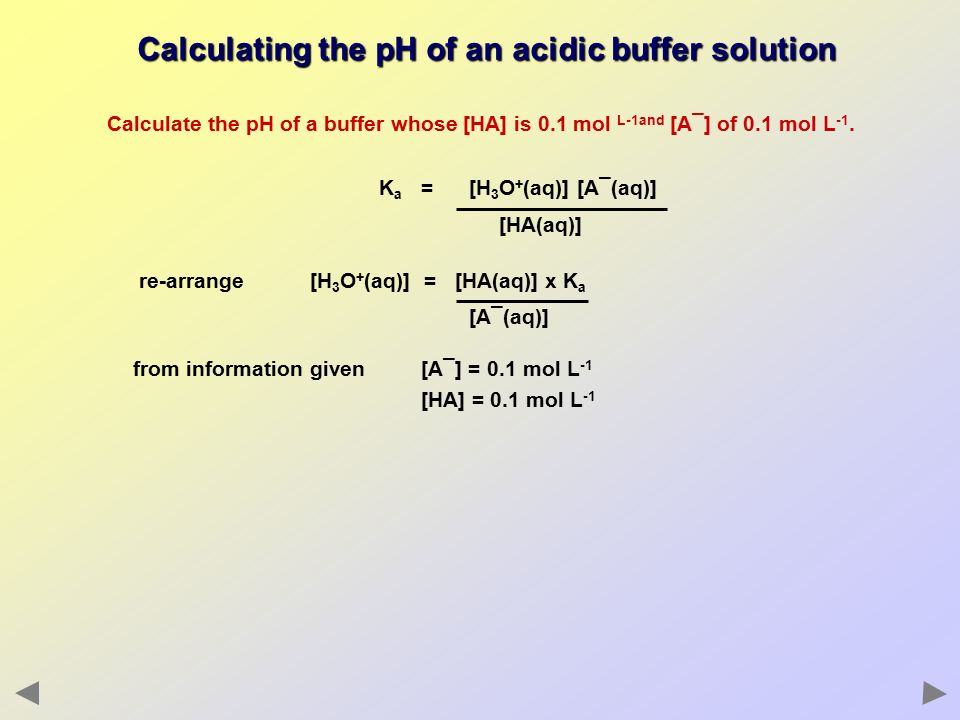
![Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ] Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]](https://i.ytimg.com/vi/t9B5VgPOTG4/maxresdefault.jpg)
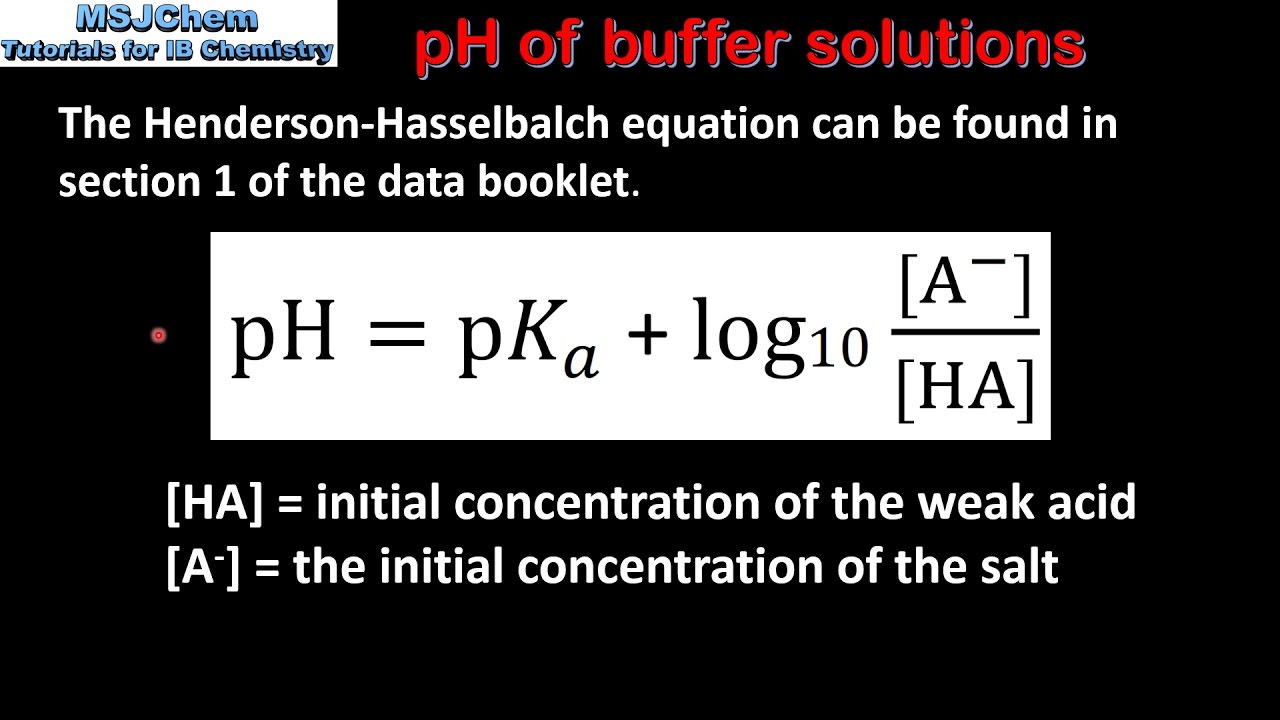
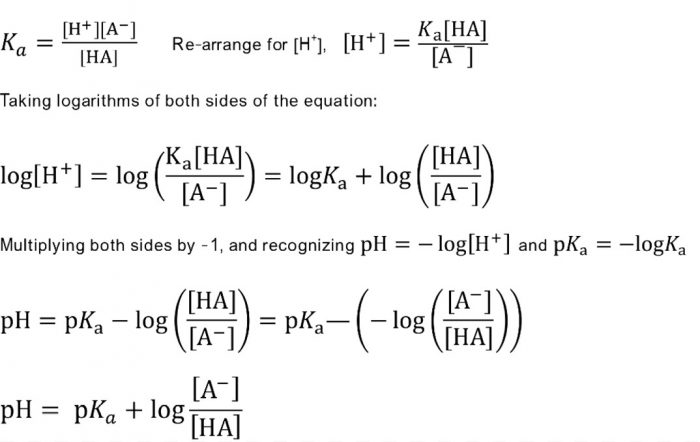
Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]

Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com

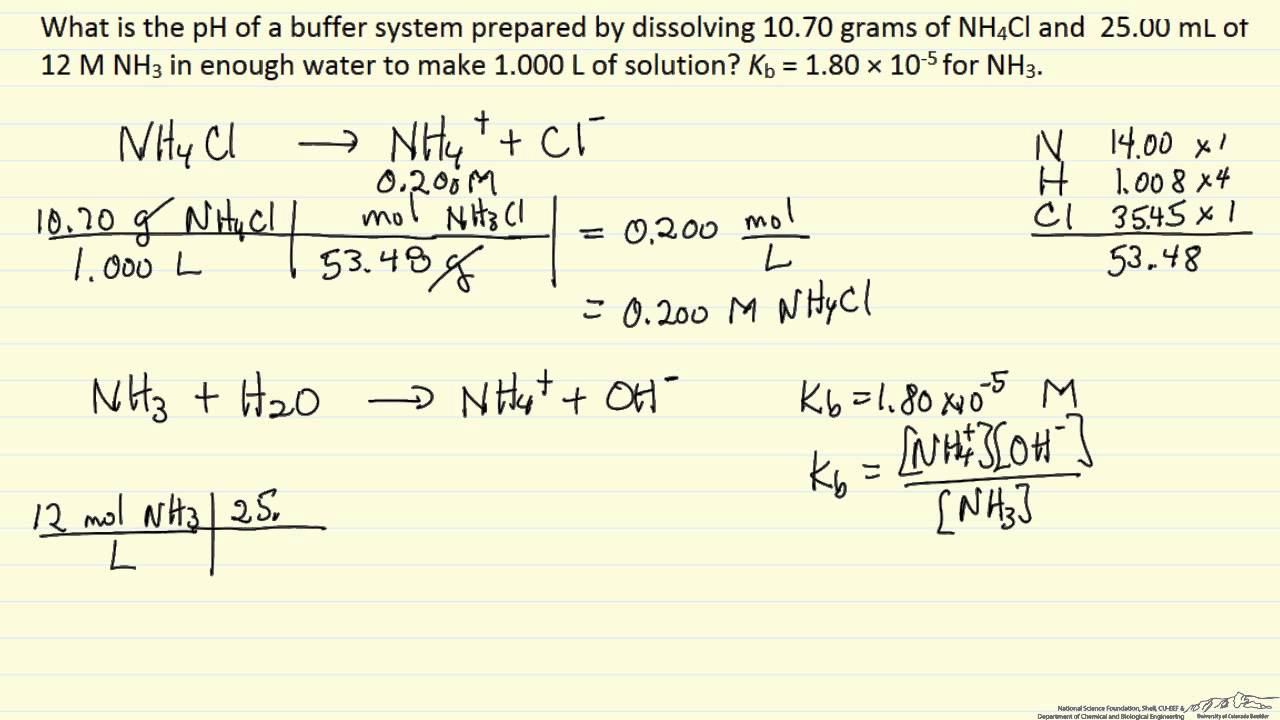
SOLVED: I V ' 07 Buffer Solution plus 6 M NaOH 1o,35 SHOW YOUR WORK FOR THE CALCULATIONS Calculate the pH of the buffer solution based on the known Ka value for

Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com